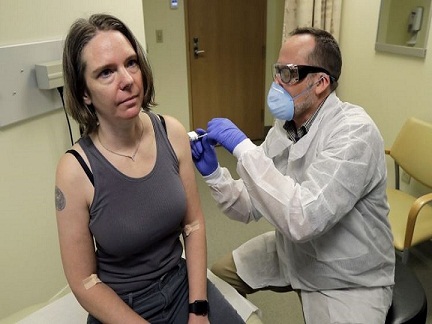
A human clinical trial of a potential vaccine for the new coronavirus began on Monday as the United States gave first shot of experimental COVID-19 vaccine to a person. As stated by the US health officials, the first human trial to evaluate a candidate vaccine against coronavirus disease 2019 has begun in Seattle.
The US National Institutes of Health (NIH) said in a statement that the open-label trial would enroll 45 healthy adult volunteers ages 18 to 55 years over approximately 6 weeks,.
As reported, scientists at the Kaiser Permanente Washington Research Institute in Seattle begin an ‘anxiously awaited first-stage study of a potential COVID-19 vaccine developed in record time’.
An operations manager at a tech company Jennifer Haller,43 of Seattle, received the injection inside an exam room.
Haller, the mother of two teenagers said it as an “amazing opportunity” for her to “do something”.
The said ‘vaccine’, codenamed mRNA-1273, was developed by the National Institutes of Health (NIH) and Massachusetts-based biotechnology company Moderna Inc.
The cases of coronavirus keep on surging across the world in which more than 1,50,000 people infected worldwide and has claimed the lives of over 7000 people.
Kaiser Permanente study leader Dr Lisa Jackson said about the experiment “We are team coronavirus now. Everyone wants to do what they can in this emergency.”
The tests came at a time when the whole world attempts to develop the vaccine for the deadly virus.
Though, these vaccines are not likely to be available for general population anytime for 12 to 18 months, said Dr Anthony Fauci of the NIH.
The President of United States Trump said that he is pleased to report that a vaccine candidate has begun the phase-1 clinical trial.